COVID-19 Pandemic in Europe:
Experiences in Switzerland, Austria and Germany
Wednesday, 14. April 2021, 14:00 – 15:30 via Livestream
This webinar was chaired by Dr Ghazaleh Gouya, Austrian member of the House of Delegates, and Dr Martin Traber, President of the Swiss Society of Pharmaceutical Medicine. Dr Marco Romano, IFAPP President, summarised the event at the end and provided an outlook for the upcoming events.
A very high level of interest for this webinar was already shown in advance with 500 clicks on the registration link, 237 registrations and 154 attendees who also took also the survey. The mean rating of the event was 4.16 in favour (out of a total of 5.00). Most of the participants came from national member associations in Germany (54), Austria (26), and Switzerland (9). The remaining attendees were from the USA and UK, with at least one person from Kuwait, Egypt, Côte d’Ivoire, Indonesia, Australia, Canada, India, Ukraine, China, and South American countries.
The presentations on the COVID-19 situation in these three countries focused on different aspects. Whilst we learned from epidemiological insights and an extensive and impressive research network in Switzerland established during the pandemic, the Austrian presenter spoke about successful collaboration in public-private partnerships and lessons learned with regard to online tools. The German speaker provided an insight into the existing body of scientific evidence of COVID-19 treatments and implementation into the German COVID-19 treatment recommendation.
1. Switzerland
Milo Puhan, Professor of Epidemiology and Public Health at the University of Zurich presented Corona Immunitas(www.corona-immunitas.ch), the national research project of the Swiss School of Public Health, a Switzerland-wide programme to assess the spread and impact of the pandemic. Fourteen universities and health organisations, more than 40 studies throughout Switzerland with 50 000 study participants.
There are repeated cross-sectional and cohort studies to determine sero-prevalence, all enrolled in a digital follow-up, and additional site-specific studies. All Swiss cantons are involved, and it could be shown that seroprevalence increased by more than twice the percentage numbers from summer 2020 to winter 2021. In early 2021, thanks to the nation-wide vaccination programme, sero-prevalence increased in the older (>65 years) when compared to the younger population. Based on a study by Zsac[1] (prospective population-based cohort of PCR+ persons) it was shown that 15% of the infected person do not develop antibodies (which was more likely after a mild course of the disease as well as in women and in smokers). Ninety % still have detectable antibodies after >6 months. The total number of infected persons is currently about 2 million in a total population of about 8.7 million.
The approved vaccines in Switzerland so far are those from BioNTech/Pfizer and Moderna. The cantons have to report a minimal required data set on the number of vaccinated persons (→15% at least once by 09th April 2021), age and sex (→around 50% of 75+ year-olds to be fully vaccinated).
Follow-up data on attitude towards the vaccinations, side effects, vaccination status by educational level, by household income, by underlying chronic disease as well as data on antibodies differentiating between infection and vaccination and more are expected throughout the upcoming year.
2. Austria
For Austria, the presentation was given by Dr Johannes Pleiner-Duxneuner, the President of the Austrian association of Pharmaceutical Medicine, GPMed, with the title “Public Private Partnerships in Times of COVID-19, The Austrian Story”.
Funding from federal ministries[2] of 26 million euros for the Corona Emergency Call were processed by the Austrian Research Promotion Agency (FFG) p in a fast-track evaluation for forty-five projects being finally awardedin the following topics in COVID-19: vaccines (2), diagnostics (18), prevention/infection control (4), prevention/protective materials (8), prevention/disinfection (3), prevention/safety (1), and therapeutics (9). Among the many projects Dr Pleiner-Duxneuner provided a few examples of research activities, e.g., with APN01, a recombinant human Angiotensin Converting Enzyme 2 (rhACE2) under Phase-2 clinical development, and Solnatide, a synthetically produced peptide.
Online events and online trainings were organized throughout the past 14 months, stakeholders from academia, industry and regulatory have come closer during the pandemic organizing multiple virtual events on Clinical trials during the COVID-19 Pandemic and other similar hot topics. An online course on Introduction to Medical Affairs and Pharmaceutical Medicine was successfully launched fully virtually and held in English in the first quarter of 2021 with planned annual courses to be held all online.
A guidance document for usage of RWD/RWE (Real World Data/Real World Evidence) is under development in a joint effort of public-private stakeholders, ethics committees and the regulatory agency. Important topics on quality criteria for RWD for primary and secondary use, the legal requirements and guardrails, overview on existing data sets/registries and which datasets to use for which analysis to be used for which analysis will be elaborated to set common recommendation on the use of RWD.
The crisis during the past months was accompanied by an enormous effort from all stakeholders in pharmaceutical medicine to collaborate.
3. Germany
Stefanie Wuestner and Sara Hogger, both at AMS Advanced Medical Services in the field of HTA (health technology assessment) preparing documents for the German AMNOG-Process (Arzneimittelmarktneuordnungsgesetz „Pharmaceuticals Market Reorganisation Act“), presented their project which is performed in collaboration withFriedhelm Leverkus from Pfizer as well as collaborators from AMGEN and Novartis. The title of their talk was ‘Good enough’? – Evaluating evidence generation for treatment recommendations on pharmaceutical therapies during the COVID-19 pandemic, dealing with the question, what kind of clinical data, had strongest impact on patient care; based on its uptake in German treatment recommendation.
We observed that clinical data with increasing level of certainty became available over time. All analysed treatments followed the following chronological pattern, since they were available early due to repurposing and compassionate use and were analysed in large platform trials:
- General treatment practice
- Observational trials, cohort studies
- Single-centre studies, interventional trials, randomised clinical trials
- Multi-centre studies, adaptive platform trials[3]
In the current project, studies of four treatments with the most comprehensive evidence from published data and guidelines available end of 2020 ([hydroxy-]chloroquine, remdesivir, anticoagulants and corticosteroids) were evaluated to understand the pattern of evidence generation and uptake into German treatment guidelines. Due to the limited time, the example of remdesivir was not presented in the webinar,
The following sources were used to generate the body of evidence for the assessment: Studies referenced in the German guidelines for hospitalized/critically ill patients (primary studies, reviews, meta-analyses) and systematic search for treatments in MEDLINE (Dec 1, 2021).
Systematic overview of the design and characteristics of the studies as well as features regarding the communication and publication of study data were shown for (hydroxy-) chloroquine (the dismissed first choice – proven to be not effective), anticoagulants (the obvious treatment based on expert consensus), and corticosteroids (the big surprise with evidence towards mortality benefit).
The response to the question “Good enough evidence?” is that coordination by a well-connected, multinational organization, early communication and cooperation with experts (e.g. senior investigators) and harmonization and standardization of study protocols and study procedures provide robust evidence and timely conclusions (good example: Meta-analysis for corticosteroids by REACT Working Group).
Evidence from large platform trials, especially with adaptive design (e.g. REMAP-CAP, SOLIDARITY) allow timely and robust conclusions and spare resources.
Take-home message of the Webinar: Work together, learn fast as the game is not yet over!
[1] ZSAC https://www.isrctn.com/ISRCTN14990068/ and Dan et al
https://science.sciencemag.org/content/371/6529/eabf4063
[2] The Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology (BMK) and the Federal Ministry for Digital and Economic Affairs (BMDW)
[3] Definition of platform trials: Master protocol, multiple treatments, flexible features, e.g., studies like SOLIDARITY, RECOVERY, and REMAP-CAP

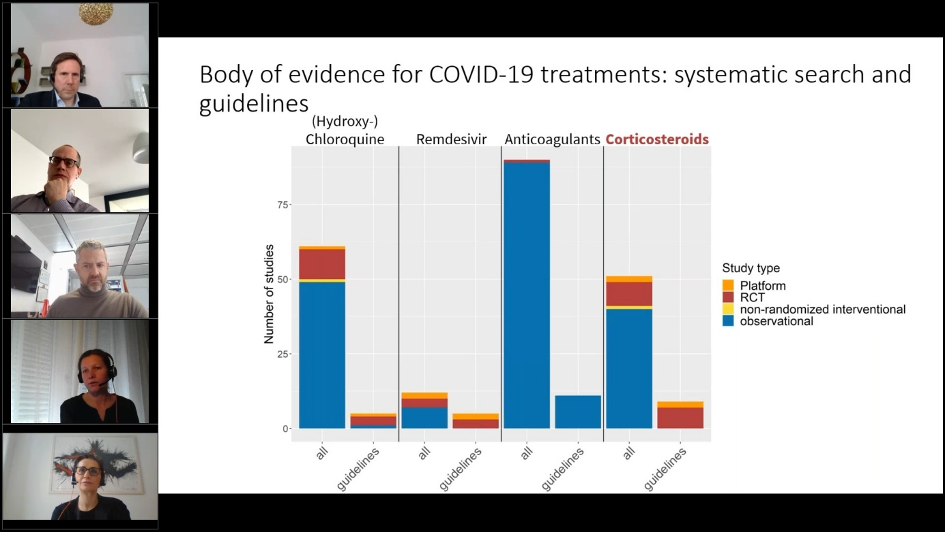
Top to bottom: PD Dr. Johannes Pleiner-Duxneuner (Roche Austria, President elect GPMed), Dr. Martin René Traber (SGPM President and Global Medical lead F. Hoffmann-La Roche AG), Prof. Dr. Milo Puhan (Professor of Epidemiology and Public Health at the University of Zurich), Dr. Stefanie Wüstner (Head of Business and Scientific Innovation AMS Advanced medical services), PD Dr. Ghazaleh Gouya Lechner (Gouya Insights, GPMed Board member). Presentation of a slide from Stefanie Wüstner on body of scientific evidence from potential treatment option in COVID-19.
